MICROGAS
SF6 MIXED
Gas for intraocular use

Initially, the only buffering agent used was air due to the strong difference in surface tension compared to water. Air, is, however, easily and quickly reabsorbed not promoting healing of the eyeball after vitrectomy surgery, so other nontoxic gaseous substances have been studied that can remain in the eye for a longer time.
Fluorinated compounds such assulfur hexafluoride (SF6), or perfluorocarbons such as perfluoromethane (CF4), perfluoroethane (C2F6) and perfluoropropane (C3F8) were selected.
MicroSF6 (pure or mixed) is a Class IIB implantable medical device. It is a high molecular weight gas used in vitrectomy, ab-external surgery, and pneumatic retinopexy. The product is intended to replace the vitreous humor and has a residence time in the eye of 1-2 weeks.
The device should be used in a sterile field, only by ophthalmologists experienced in vitreo-retinal surgery.
The MicroSF6 is inserted into a compact bubble at the end of the vitrectomy procedure after a fluid exchange with air. The gases contained in the intraocular bubble go into solution with the fluids adjacent to it and leave the eye over time, diffusing into the bloodstream. In contrast, gases contained in the blood stream enter the bubble regulated by the partial pressure of each gas in its environment.
Then nitrogen and other air components diffuse toward the bubble containing SF6, while SF6 will diffuse toward the blood.
Fundamental is the speed of diffusion; while SF6 will exit the eye slowly, the faster the nitrogen dissolved in the blood will enter the bubble since SF6 has a molecular weight 5 times greater than nitrogen. This results in an expansion of the gas bubble in the eye by acquisition of nitrogen from the blood, up to a maximum beyond which, the nitrogen pressure now being equal to that of the blood, it can only be reabsorbed. This behavior allows the bubble to remain longer in the eye, but with the drawback that it can cause hypertone. The introduction of a bubble of gas mixed with nitrogen reduces the diffusion rate of nitrogen, preventing hypertone.
MICROGAS SF6 MIXED
MicroSF6 (pure or mixed) is injected into the vitreous chamber for the purpose of replacing the vitreous and promoting retinal adhesion. During the average residence time in the eye (about 1-2 weeks) the gas is gradually replaced by aqueous humor and exhaled.
PRODUCT ASSORTMENT
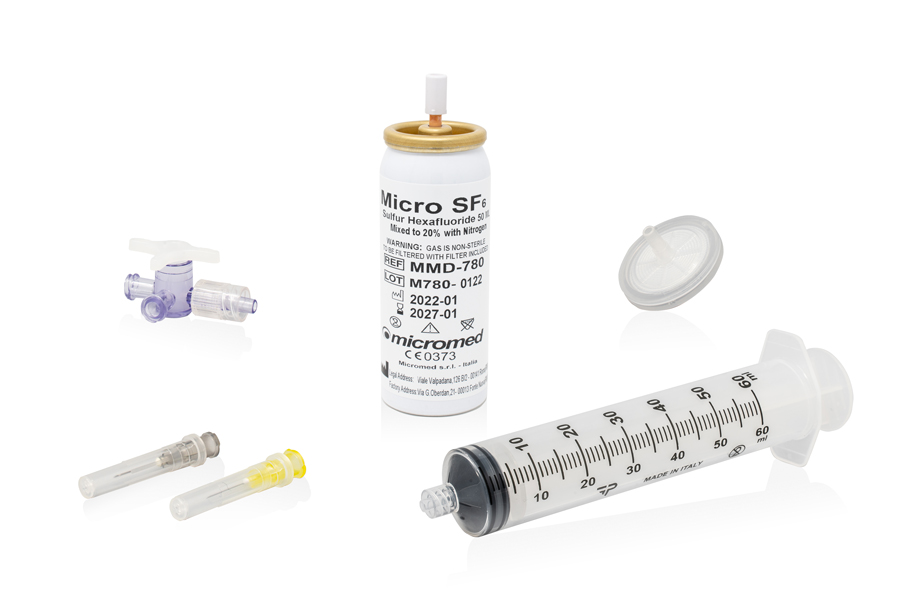
The MicroSF6 Mixed device is available in one variant in which SF6 is mixed with 20 percent nitrogen. Besides the gas canister, the kit consists of a Connector, a 0.2 μ Sterilizing Filter, a 60 ml PP Syringe, a 3-way Tap, a 27 G Needle and a 30 G Needle.
|
CODE |
DESCRIPTION |
NAME |
|
MMD-780 |
Canister pre-filled with SF6 Gas mixed with 20% nitrogen and Connector, 0.2 μ sterilizing filter, 60 ml PP syringe, 3-way tap, Needle 27 G AND Needle 30 G |
MICROSF6 Mixed |
VIDEO TUTORIAL.
How to use: Before infusion, the MicroC3F8 gas should be sterilized by passing through the filter, the other accessories included in the package are already sterilized at ETO. In the case of pure MicroC3F8, it is necessary to prepare the mixture with air before proceeding to the infusion procedure, this is done directly in the syringe provided, carefully following the directions in the bugiardine. In the case of premixed MicroC3F8, however, simply fill the supplied syringe with the gas contained in the canister and proceed directly to use.
MicroC3F8 has an average residence time in the eye of no more than 28 days