PDMS is used for long-term (at least 30 days) ocular tamponade after vitrectomy surgery.
PDMS undergoes scrupulous biocompatibility testing before packaging, and the chemical analysis performed allows us to guarantee high purity of the silicone oil due to the presence of a very low percentage of volatile chains (0.1%).
This product line was approved for marketing in 1999 and is a Class IIB medical device.
Silicones have been used in the medical field for about fifty years. No other material is sufficiently biocompatible, reliable, flexible and easily sterilized as silicone.
Liquid PDMS is indicated and recommended in all world pharmacopoeias for use as a medical device. It is odorless, tasteless, does not support bacterial growth and does not react with other materials, but most importantly, it has high compatibility with human tissues and body fluids.
Several product categories belong to the PDMS family, which differ from each other based on the primary packaging and viscosity of the silicone oil mixture.
Micromed PDMS is marketed in different viscosity variants:
PDMS 1000 characterized by the presence of chain lengths with a standard deviation less than 0.02% and a typical viscosity of 1000 centiStokes; its braking index is conventionally set at 1.
The PDMS 1300 centiStokes with the same characteristics but with a 4% higher braking index.
PDMS 2000 has a braking index greater than 15 percent and is indicated in cases where emulsification with protein fluids in the eye is likely to occur independent of surgery.
The latest variant is the PDMS 5000 which has a braking index greater than 70 percent and is indicated in cases where retinal manipulation has been significant, such as in the case of strong overhanging treatments on major cerclages and for more than 40 percent of the retinal surface, or in the case of major retinotomies in the presence of cerclage or large retractions.
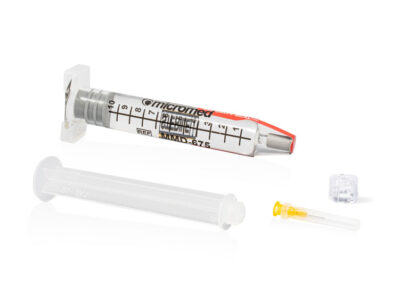
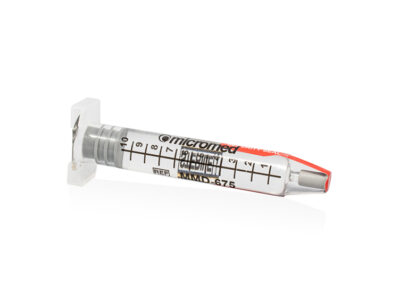
PDMS GLASS SYRINGE
Polydimethylsiloxane (PDMS) is a highly viscous oily liquid whose high purity and transparency allows long-term temporary replacement of the vitreous body.
PRODUCT ASSORTMENT
The Micromed PDMS Glass Syringe device consists of a glass syringe pre-filled with 10cc of silicone oil (1000, 1300, 2000 and 5000 cSt), the kit is completed by the presence of a luer lock adapter and a 23g cannula.
|
CODE |
DESCRIPTION |
NAME |
|
MMD-665 / MMD-665A |
10 cc glass syringe preloaded with pdms 1000cst luer lock adapter cannula 23g |
PDMS 1000 Glass Syringe |
|
MMD-675 / MMD-675A |
10 cc glass syringe preloaded with pdms 5000cst luer lock adapter cannula 23g |
PDMS 5000 Glass Syringe |
|
MMD-686 / MMD-686A |
10 cc glass syringe preloaded with pdms 1300cst luer lock adapter cannula 23g |
PDMS 1300 Glass Syringe |
|
MMD-696 / MMD-696A |
10 cc glass syringe preloaded with pdms 2000cst luer lock adapter cannula 23g |
PDMS 2000 Glass Syringe |
VIDEO TUTORIAL.